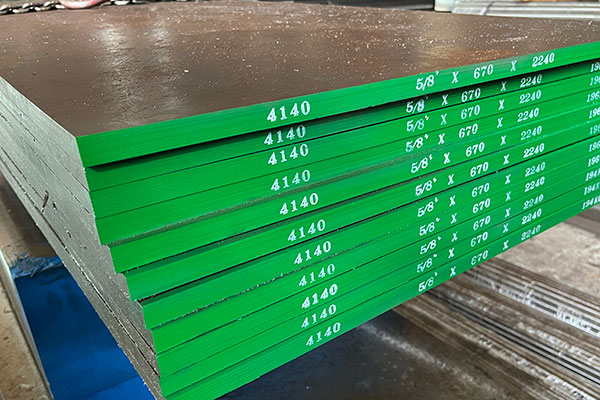
Carbon element plays a key role in balancing strength and toughness in steel. Reasonable control of carbon content is a core link in steel design and production. The higher the carbon content, the higher the hardness of the steel, but the worse its plasticity and toughness. When the carbon content exceeds 0.23%, the welding performance of the steel deteriorates. Therefore, the carbon content of low-alloy structural steel used for welding generally does not exceed 0.20%. High carbon content will also reduce the atmospheric corrosion resistance of steel, and high-carbon steel in open-air stockyards will easily rust. In addition, carbon can increase the cold brittleness and aging sensitivity of steel.
Carbon exists in two forms in steel. One is free state, such as iron-carbon solid, amorphous carbon, annealed carbon, graphite carbon, etc., which can be directly represented by “C”. The other is combined carbon, that is, carbide of alloy elements, such as Fe3C, Mn3C, etc., which can be represented by “Mc”. The former generally cannot react with acid, while acid can dissolve and destroy the latter. In steel, combined carbon is the main form, and free carbon only exists in iron and annealed high-carbon steel. In component analysis, usually measurethe total carbon content .
 The specific role of carbon element in steel:
The specific role of carbon element in steel:
Effect on the microstructure of steel: The carbon content determines the microstructure of the steel, such as the proportion of pearlite, bainite or martensite, which in turn affects the overall performance of the steel.
Effect of heat treatment on steel Hardenability: Steel with high carbon content is more likely to form martensite during heat treatment (such as quenching), significantly increasing the hardness. Carbon also affects the phase transformation temperature and hardenability of steel, which determines the final properties of the steel after heat treatment.
Effect on the mechanical properties of steel Enhanced strength and hardness: Increased carbon content will significantly increase the strength and hardness of steel. This is because carbon atoms form carbides (such as Fe3C) in the iron lattice, which enhances the steel’s ability to resist deformation.
Reduced ductility and toughness: Although carbon increases strength and hardness, too high a carbon content can reduce the ductility and toughness of steel, making it more susceptible to brittle fracture.
In the past few articles, we also analyzed the role of Cr in steel and the relationship between Ni and steel. If you are interested, you can take a look.